B cells constitute an essential part of the adaptive immune system, by producing antibodies that neutralize toxins and target infected or malignant cells for destruction. B cells recognize pathogens, or pathogen-derived antigens by their characteristic B cell receptor (BCR), which then triggers intricate downstream signaling pathways ultimately leading to cell differentiation and antibody production. Tightly regulated, yet robust triggering of B cells is critical for mounting of humoral immune response upon infection or vaccination. Diminished B cell activation leads to a variety of immune deficiencies and, on the other hand, lowered activation threshold can lead to autoimmune disorders. Furthermore, transformations in the B cell signaling pathways may lead to malignant growth and development of lymphoma. In vivo, B cell recognizes antigen (Ag) either in soluble form or displayed on the surface of antigen presenting cells (APCs). An important event following BCR signaling is the uptake, intracellular vesicular trafficking and proteolytic processing of the BCR-bound antigen into peptides on MHCII complexes. These complexes are then presented to the cognate T cell counterparts to help initiating a cascade a B cell differentiation into high affinity antibody production and immunological memory.
Antigen processing pathway
The costimulatory signals received from the interacting T helper (TH) cells are critical for B cell differentiation into plasma cells that specialize in secreting high-affinity antibodies, with the specificity dictated by the BCR. However, to receive T cell help, B cells need to internalize the encountered antigen and process it into peptides for loading on class II Major Histocompatibility Complexes (MHCII) for cell surface presentation to cognate TH cells. One of our goals has been to unravel the mechanisms of the antigen vesicle traffic to increase our understanding on the spatio-temporal regulation of the antigen processing pathway in B cells. In this project, we employ various 3D imaging techniques, including live imaging and super-resolution modalities such as SIM and SRRF, and have also set up a new technique to improve the visualization of the freshly internalized cargo vesicles (SHIP; Hernández-Pérez & Mattila, 2022). We have demonstrated high heterogeneity in the antigen trafficking vesicles and our data suggests that the antigen is directed into processing-compatible vesicles in a very fast and directed manner, together with the surface-derived MHCII that could facilitate early peptide-MHCII presentation (Hernández-Pérez et al., 2020). In our current projects, we continue to unravel the peculiar endo-lysosomal vesicle populations in B cells to better understand their functions in B cell activation, antigen presentation and beyond. Furthermore, we are interested in understanding the extracellular vesicle release in the B cell activation process and are now characterizing this phenomenon in detail.
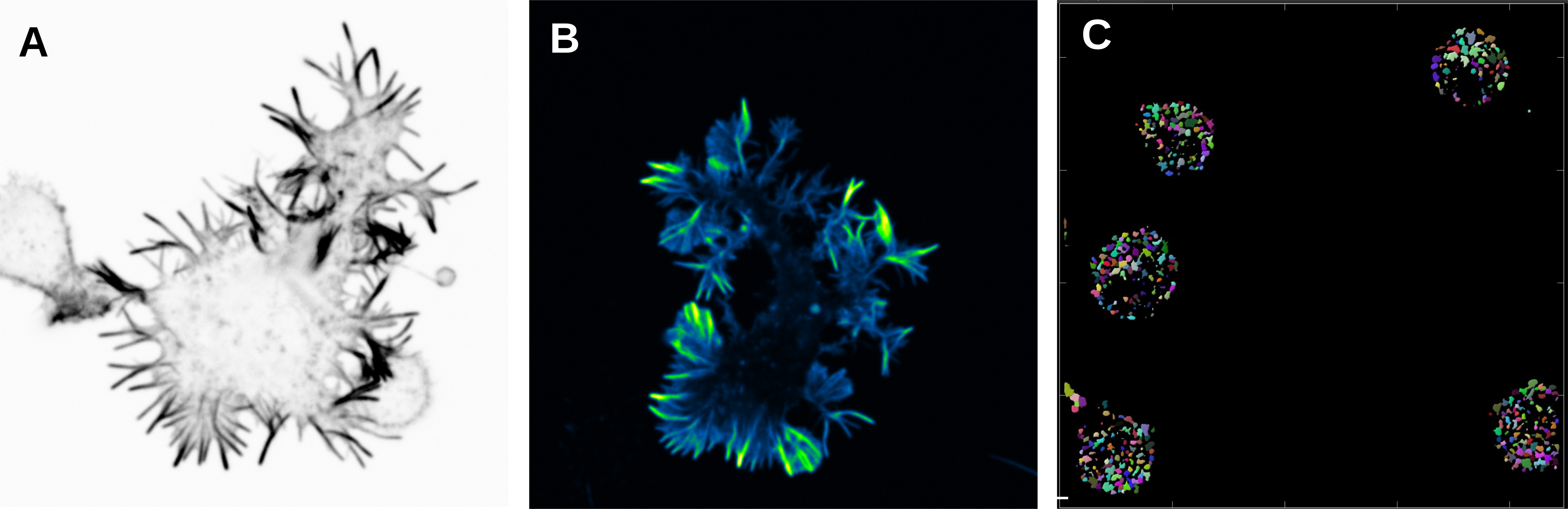
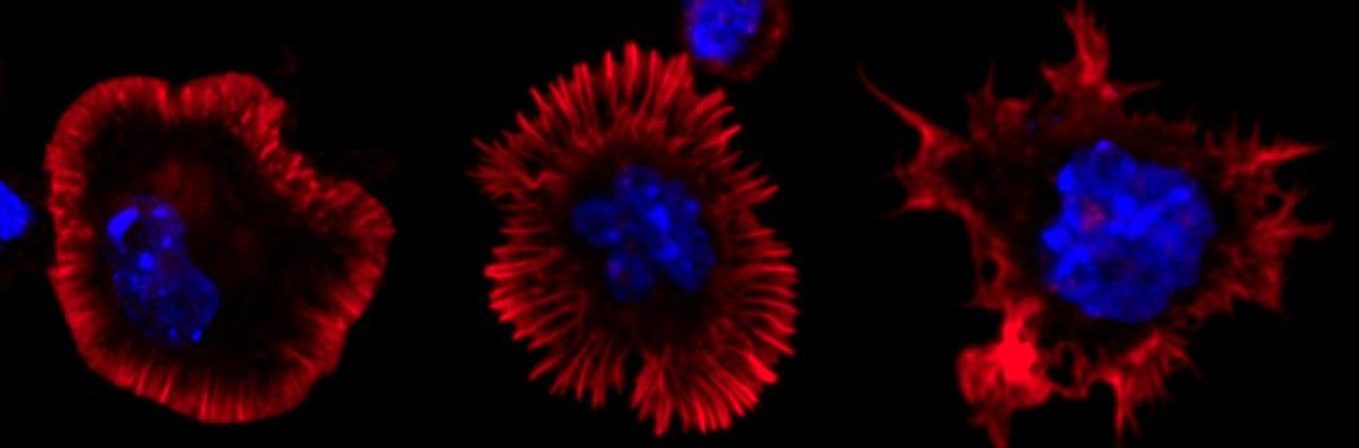
A) and B) B cells activated in anti-IgM (surrogate antigen) coated glass and stained with Phalloidin. Spinning disk confocal microscope. C) B cells activated with antigen and stained for EEA1 (early endosomal marker). EEA1-vesicles were detected in Imaris with surface rendering and number of vesicles was quantified. Different colours show different vesicles in one cell.
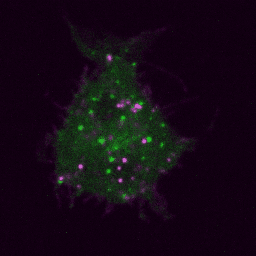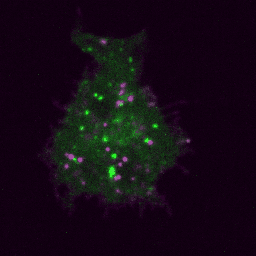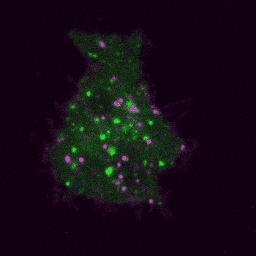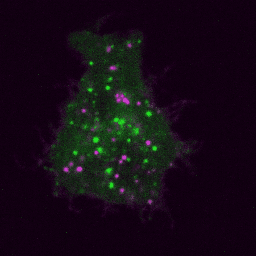
Live imaging (spinning disk confocal microscope). Time-lapse of B cell transfected with GFP-Rab5 (green) and activated with surrogate antigen (magenta).
Proteomic approaches to reveal intracellular dynamics of BCR activation
We are interested in understanding the nature of the multibranched dynamic intracellular responses downstream of BCR following activation with either soluble antigen and antigen displayed on the surface of antigen presenting cells. For that, we are focusing on two different approaches:
- APEX2, a well characterized proximity labelling technique, allows the identification of proteins that reside in the close vicinity of the BCR during the different stages of cell activation by soluble antigens.
- B cell activation by antigen tethered on magnetic beads (mimicking APCs), which induces the formation of a structure called immunological synapse. This will allow us to investigate the most prominent form of B cell activation in vivo, as the proteins recruited to the immunological synapse site will be extracted and analysed via mass spectrometry.
With this project, we intend to depict and uncover novel proteins and pathways that would bring better understanding to the various molecular machineries associated with BCR activation such as signalosome formation, antigen internalization, vesicular traffic of antigen processing, and cytoskeletal remodelling.
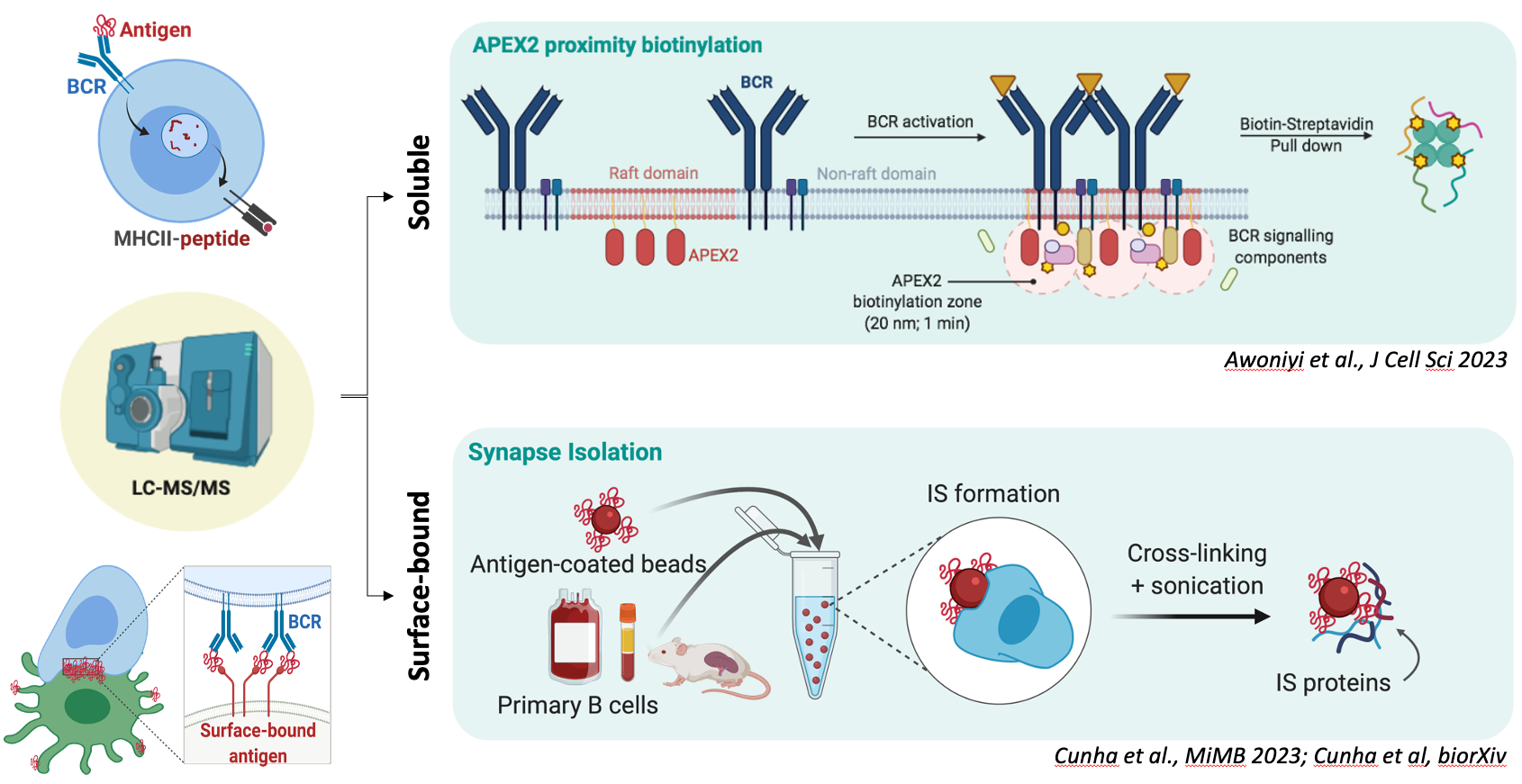
Experimental design of the proteomic approaches taken to study B cell activation. On top, B cells expressing lipid raft-directed APEX2 are used to track the cellular events occurring at the vicinity of the plasma membrane after engagement of the IgM BCR. APEX2 proximity biotinylation supports high spatial and temporal resolution. Below, isolated primary B cells are conjugated with magnetic beads coated with surrogate antigens to induce the formation of the immunological synapse. The extracted cell adhesion (immunological synapse) is then directed for proteomic analysis. Schematic created using BioRender
Yellow fever virus (YFV) processing by B cells
In Mattila lab, we are also interested in the BCR-mediated internalization of viruses and viral antigens, as well as the subsequent antigen processing for presentation to T cells. As part of the Horizon Europe-funded Yellow4FLAVI consortium, we are investigating the underlying mechanisms of yellow fever virus (YFV) and YFV vaccine 17D-induced immunity. We are focusing on intracellular trafficking of the wild-type and 17D for peptide presentation in B cells. The goal of the Yellow4FLAVI is to uncover the mechanisms that drive the success of the YFV vaccine and thereby contribute to the future design of effective and safe vaccines for other members of the flavivirus family.
More information: https://www.yellow4flavi.eu/

17D infected VeroE6 cells stained with E-specific antibody and DAPI.
AutoCoEv, an automated computational pipeline to predict protein interactions
With the aim to find new ways to analyse large protein datasets, like the ones coming from proximity proteomic screens, for possible protein-protein interactions, we have set up a new unbiased tool based on coevolution. AutoCoEv is an automated pipeline to analyse protein datasets for potentially coevolving pairs to predict new interactions or connections between proteins. You can find AutoCoEv with a virtual machine and a good manual on our GitHub pages. Please, let us know if you wish to give it a go and so can help you out!